Conflict of Interest (COI) Questions/Answers
Investigators and research staff should understand the organization’s conflict of interest policy in order to follow it. For example, investigators should know what interests the organization requires to be disclosed. Investigators and research staff should know how, when, and to whom to disclose interests.
Answers
What is UK's policy on Conflict of Interest (COI)?
UK actually has two policies on conflict of interest; one for research investigators and one for the institution itself.
Financial COI related to research of individual investigators is covered in Administrative Regulation (AR) 7.2 - Financial Conflicts of Interest in Research. The AR outlines procedures for defining, identifying, disclosing, managing, reporting and training regarding COI.
A potential or actual Conflict of Interest (COI) exists when a significant financial interest (as defined below) of an Investigator or a family member of the Investigator could directly and significantly affect the design, conduct, or reporting of research.
COI is administered by the Office of Sponsored Projects Administration (OSPA).

See the OSPA COI website for guidance.
If you have questions or need assistance with a specific situation, contact Conflict of Interest Administrator Emily Bradford at 257-9420 or emily.bradford@uky.edu.
ORI & OSPA coordinate handling of Investigator COI for both funded and unfunded human subject research.
How is researcher COI managed?
The IRB application asks if any investigators or key study personnel have a Significant Financial Interests requiring disclosure and if the interests are related to the proposed research?
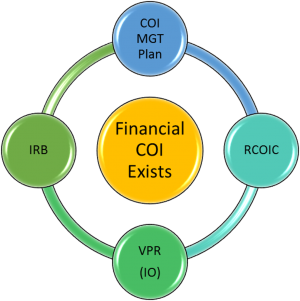
- If a financial COI exists and cannot be eliminated, the investigator works with Emily Bradford to complete the University of Kentucky template management plan and obtains approval from their Associate Dean for Research (ADR).
- All management plans are referred to the Research Conflict of Interest Committee (RCOIC) for review.
- The RCOIC recommends a plan which is submitted to the Vice President for Research (VPR) for approval.
- The IRB does not complete its review and approval of the IRB application until it receives the final VPR-approved management plan. The IRB may not change the approved plan, but it may impose additional restrictions/conditions on the protocol (e.g., disclose conflict in the informed consent document).