E-IRB News 2021

June 23, 2021
Automated System Reminders [HTML]
For customer service purposes E-IRB was designed to automatically disseminate e-mail reminders to researchers at key points in the life cycle of an application. This includes, but is not limited to, when the application is returned to the researcher because either ORI or the IRB has requested additional information or revisions.
In response to recent feedback, ORI has made a change to decrease the period of time that lapses between automated reminder notifications after an Initial Review (IR), Modification Request (MR), or Continuation Review (CR) application has been returned to the researcher. This means, for example, if the researcher submits an Initial Review and it is returned for revisions, instead of E-IRB issuing an email reminder three months from the time the application was returned, it will now send the reminder 21 calendar days from the time the application was returned.
ORI understands there are circumstances that prevent a researcher from being able to respond before email reminders are disseminated. While ORI will not be able to stop the notifications from being issued if a response has not been submitted in E-IRB, researchers should reach out to the ORI staff managing the application in question to let them know if circumstances require more time to respond.
April 6, 2021
Application Section Comments [HTML]
On each submitted IRB application or one pending review/approval, users will see a “View Comments” panel on the right-hand side of the page listing unresolved comments for that section (note: the panel is disabled on unsubmitted, approved, and inactive/closed applications).
This panel allows comments to be visible in each section as the user works through the application to provide a response or complete a review.

When in the “View Comments” panel:
- Users have the ability to indicate whether a comment has been resolved. If the comment has been marked resolved, the resolved comment will display under a Resolved Comments tab to the right after the panel has been refreshed (e.g., Press F5 on your keyboard).
- If you have submitted your application and are awaiting ORI/IRB review, please refrain from adding or editing existing comments and/or changing the resolved/unresolved status of them.
- The user has the option to collapse the “View Comments” panel by clicking the pin icon in the upper right corner of the panel.

- When the panel is collapsed, click the vertical “View Comments” tab to expand the panel.

- If the user leaves the section in which the Comments panel was collapsed, the system doesn’t remember the setting and the user will need to click the pin icon again to collapse the panel, if desired.
- Any user (Researcher, ORI, IRB) can indicate a comment has been addressed by clicking the “resolved” checkbox. Likewise, any user can click the “resolved” checkbox to remove the check mark if the comment is deemed not truly addressed/resolved. There might be restrictions implemented at a later date regarding who can use the “resolved” checkbox.
Adding Section Comments:
- To add a comment, click the “+ New Comment” button; in the window that opens insert the comment and click “Add Comment” to save it.

- You can only edit a comment you personally authored/ inserted. Use the blue “Edit” link to the right of your name to make an edit.
Application - "All Comments"

- Implementation of the features in the “View Comments” panel has not yet altered the application’s “All Comments” window (accessed from the Application Links menu on the left) except for an additional column indicating “true” or “false” for whether the comment has been resolved.
March 26, 2021
Exempt Applications: Early Closure Requests [HTML]
The researcher and ORI have the ability to close an exempt (XX) application prior to the six year certification end date. This may be necessary for reasons like if a PI is leaving UK (or has left), or the research activities have been completed and the PI does not want the application open until the end of the six year certification date.
To start an XX closure request, select “Request Study Closure” located in the menu on the left of the approved application’s landing page.
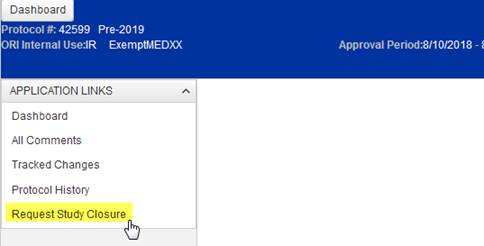
Upon selecting “Request Study Closure”, the user is prompted to confirm the closure request (or cancel if accidentally selected).
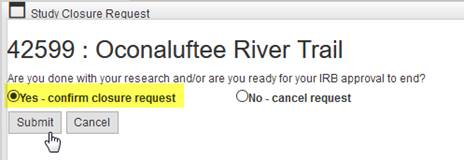
If the user confirms the XX closure request, ORI will receive the request like they would for an administrative study closure (as an Other Review). The user cannot create more than one study closure request; a message will appear on the Study Closure Request pop-up window to block the user from doing so. If “cancel” is selected, nothing happens to the application -- the closure request pop-up window just closes.
If ORI should require additional information prior to processing the closure request, the researcher will be prompted with a “Modify Reportable” task on their Dashboard (under the Other Reviews tab of their Inbox). When the researcher clicks “Modify Reportable” the Review Notes can be read to identify what additional information is required, and a response can be inserted on the page:
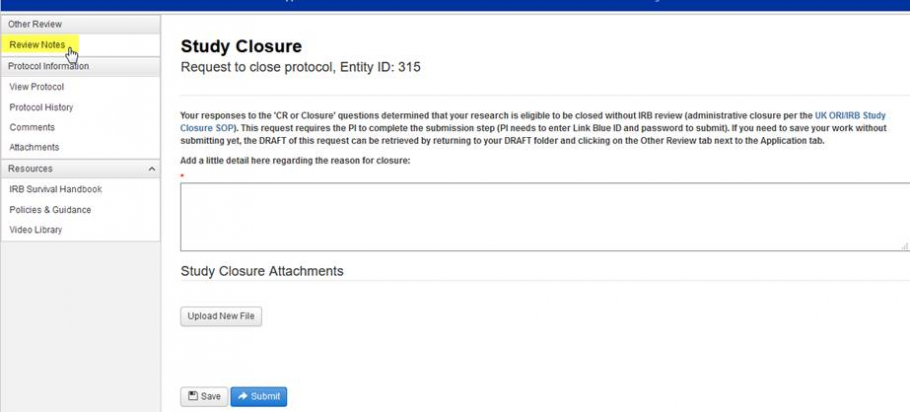
When the XX closure request has been completed by ORI:
- an automated E-IRB email is disseminated to the researcher as notification of completion of the closure;
- the application is located under the researcher’s Inactive folder; and
- an official closure letter goes on the application’s record (see application’s Protocol History).
Questions about the exemption closure process can be directed to the ORI Staff who have been managing your application (for Medical Exempt reviews, it will be either Joe Brown, Jenny Smith, or Jill Marion; for Nonmedical Exempt reviews, Lori Miller, Daniel Ehrlich or Craig Vaughn).
February 17, 2021
Protected PDF Files in E-IRB [HTML]

As the research community has acclimated to an electronic IRB submission and review system over the last couple years (E-IRB), this progression inadvertently, albeit naturally, includes researchers uploading PDF attachments that retain some level of protection or security (e.g., for verification of e-signatures; password protection for proprietary content).
For ease of use, E-IRB was designed to allow the user to click one time on a PDF file that combines all the materials submitted for an Other Review (e.g., Protocol Violation; Deviation/Exception request; Unanticipated Problem). However, digitally signed, password protected, or other secured PDFs cannot be combined with other PDF’s where some level of protection applies by design, so the user gets an error message when clicking on the link (“View # __ ) to open that Other Review.
ORI and Research Information Services (RIS) understand protected PDF attachments are a desirable component of the submission process. However, the technology does not fit our process, so RIS is devising an alternate means of accessing Other Reviews. This change in design will require a user to click on each document associated with the Other Review in order to open it. E.g., The screen shot of the web page form filled in; any attachments included with the submission; and when applicable, the IRB approval/acknowledgement letter. While this may result in the need for a couple more clicks, it will also eliminate any breaks in the system, subsequent need for IT/ORI intervention, and ultimately streamline the submission and review process.
Until RIS has implemented this design change, please bear in mind that uploading a PDF with signature certification, password protection, or other security setting, may slow down or prevent the submission review process for Other Reviews and for application reviews.
As always, thank you for your patience as we do our best to improve E-IRB!