Our technology commercialization process follows best practices in technology transfer, licensing and new venture development. Utilizing years of successful licensing and start-up experience, our efforts are collaborative with innovator input and involvement. Please reach out to our technology commercialization team to learn more about how we can help accelerate industry adoption of your innovation.
Resources for UK Innovators
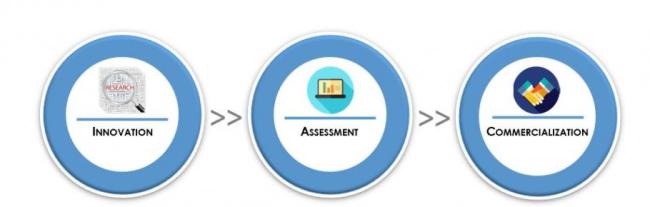
Technology Commercialization Process
1. Innovation
- Innovator researches and discovers innovation
- Innovator discloses innovation to TC
2. Assessment
- TC completes a technology and market assessment of the innovation
- TC pursues appropriate IP protection
3. Commercialization
- TC develops and implements a commercialization strategy for licensing the innovation to a start up or to an established company
Submit an Innovation
Why File an Innovation Disclosure Form?
- The Innovation Disclosure Process provides the OTC with basic information about the innovation, allows us to assess patentability and commercial potential, and supports the patent filing decision. As seen below, the process includes 3 easy steps.
- Once your innovation or discovery has successfully completed all three of these steps, the OTC will begin working with you to commercialize your innovation via an industry license, a startup company or other means.
- We strongly encourage innovators to submit an Innovation Report Form before any public disclosures (lectures, posters, presentations, meetings with industry, etc.) that may adversely affect the patentability of an innovation. However, even if you have already disclosed your idea, it is still possible to receive protection, so please contact us anyway!
Innovation Reporting in 3 Easy Steps
- Step 1: Click here to submit an Innovation Disclosure Form and log in using your Linkblue ID email and password. If you have issues logging in, send an email here. *See below for materials needed for the Innovation Disclosure Form. Watch a walk-through of the process with descriptions of the field.
- Step 2: Complete all relevant areas of the form.
- Step 3: Once completed, click Submit.
Materials Needed:
- Home and University mailing addresses for each UK Innovator
- LinkBlue ID and Employee ID (required for all UK Innovators contributing to the innovation)
- Contribution percentage for each innovator
- Complete description of the innovation (ability to attach documents)
- Funding information (sponsor, grant number, principal investigator, award date)
- Information on how your innovation differs from existing technologies, innovations, etc
View Your Innovations
UK Innovators can view past University of Kentucky innovations they are inventors on through the Sophia system. Contact the Intellectual Property/Compliance Coordinator to request access to Sophia.
Login to the Sophia system using your Linkblue account.
Access all of your innovation reporting history using TC's online portal. The portal can be accessed using your Linkblue login. If you have any issues with your innovation account views, please contact the Intellectual Property Coordinator.
Income Distribution Process
License and royalty income derived from the commercialization of UK inventions is distributed according to UK's Administrative Regulations as follows:
- Income Received from Licensee
- Income Distributed to Inventors (40%)
- Following payment of patent and/or other expenses, distributions occur by the end of December and June of each fiscal year
- Income Distributed to Colleges (20%) and Departments (20%)
- Distributions occur by the end of July and January of each fiscal year
Request an MTA/NDA/DUA
About Material Transfer, Non-Disclosure and Data Use Agreements
-
CONTACTS:
Clinical Trial NDAs: Jessica Heskel, jessica.heskel@uky.edu
DUAs and MTAs from College of Medicine: Ali Bocook Yankey, ali.yankey@uky.edu
Research NDAs and MTAs (all colleges except College of Medicine): Nina Amster, nina.amster@uky.edu - Material Transfer Agreement (pdf, 1pg)
- Confidential Disclosure Agreement (pdf, 1pg)
Biological Materials Form
Biological materials such as cell lines, antibodies, plasmids, etc. constitute tangible intellectual property within the scope of UK’s IP Policy (Administrative Regulation 7:6). Although these materials are not typically patentable, they may be commercialized.
It is important to complete a new biological materials form to enable us to better understand what the material is, identify who created it, and assess its commercial potential. Additionally, we must be sure to identify any grants or sponsored awards used to support the development of the materials to ensure our compliance with those contracts
To report new biological materials you have developed, please contact Technology Commercialization to request our Biological Materials Report form.
Start a Company
Researcher Agreements and Contracting
General Information
- Signature Authority
- Memorandum of Understanding
- Handling Confidential Information
- Types of Confidential Information
- Intellectual Property
- Research Data Retention & Ownership
Frequently Asked Questions (FAQs) for the Innovation Disclosure Process
Why should I disclose an innovation to Technology Commercialization?
As University of Kentucky employees and researchers, we all have a duty to disclose new innovations. In addition, UK OTC can assist with the commercialization and dissemination of your innovation, and UK OTC ensures all federal compliance regulations are met with respect to federally-funded projects.
When should I disclose an innovation to Technology Commercialization?
As soon as your innovation has moved from an idea to an actual embodiment of your innovation.
Do I need to have a working prototype to disclose an idea?
No, a prototype is not required for filing.
What if I have published or presented my idea before submitting a disclosure?
Although this could affect intellectual property rights, you should still disclose your innovation to TC, as protection may still be available.
Note: It is best to submit an innovation disclosure form before releasing it in a public venue, including UKNow or websites.
Who should I contact if I have additional questions or want to discuss my idea before submitting an innovation disclosure form?
Send an email to otcinfo@uky.edu.
Should I complete a form if I would like to get a copyright or trade secret?
Yes, the disclosure form can be used for all forms of intellectual property.
I don’t think my idea is patentable, should I still complete an innovation disclosure form?
Yes, there may be other forms of intellectual property available for the innovation.
Should I include data when submitting an innovation disclosure form?
All data related to your innovation should be included in the disclosure. Our online disclosure form allows for the attachment of documents to help facilitate this.
Should I complete an innovation disclosure form if I collaborated with someone from another university?
Yes, a disclosure form should be submitted to Technology Commercialization in any collaboration resulting in an innovation. In addition, your collaborators should also submit a disclosure to their respective technology transfer offices.
How should I disclose an innovation to Technology Commercialization?
You should complete the Technology Commercialization electronic innovation disclosure form. You can access this form in a few ways:
- Click on 'Submit an Innovation' on the navigation bar on the Technology Commercialization website;
- Click on 'For UK Innovators' on the Technology Commercialization website navigation bar, which provides instructions, FAQs and the link to Submit an Innovation;
- Log in to Sophia (Intellectual Property portal) with your Linkblue ID email and password and click on Innovation in the upper right-hand side; or
- click here.
Who should I contact if I am having issues logging in or submitting the innovation disclosure form?
Send an email here.