UK study will look at unintended consequences of classifying controlled substances
Researchers’ reasons for launching a study are sometimes purely intellectual: They may be intrigued by an inexplicable observation or challenged by a compelling question.
Other times, the spark may be deeply personal.
Such was the case for University of Kentucky doctoral student Kara Cook.
Darren’s story
Cook’s brother-in-law, Darren Noble, suffered from a chronic autoimmune disease called Sjögren’s syndrome. Symptoms of this rare condition can include dry mouth and eyes, fatigue and muscle and joint pain.
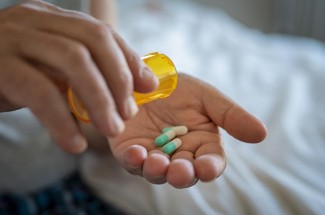
In Darren’s case, the pain increased to the point where it became nearly intractable. Only opioid analgesics were up to the task of managing his pain.
During the course of Darren’s treatment, stricter quantity limits were placed on opioid prescriptions. As a result, he could no longer receive an adequate supply of pain medicine. Under his doctors’ care, Darren tried steroids and other drug therapies as well as surgical treatment, but nothing could quash his chronic pain.
Eventually, his doctors admitted him to a hospice program. With hospice’s focus on palliative care, Darren could once again receive doses of opioid medications that were safe and effective for him.
Then Darren came down with COVID-19. Given his already compromised health, the COVID-19 symptoms rapidly grew worse. Overcome with fear that a trip to the hospital would make him ineligible for hospice care and the medicines that made his life bearable, Darren deferred in-patient care.
Days later, he died of complications of the COVID-19 infection.
“Drug scheduling is intended to protect people from the most harmful drugs," Cook said of classifying drugs based on their misuse potential and restricting access depending on a drug’s classification. "Scheduling is meant to make our lives safer, our lives better. In my brother-in-law’s case, increased restrictions on opioid prescribing made his life worse and may have contributed to his death.”
And so the seed was sewn.
Nonmedical Gabapentin use in Kentucky
Shortly after, Cook and her adviser, Assistant Professor Rachel Vickers-Smith, Ph.D., of the UK College of Public Health’s Department of Epidemiology and Environmental Health, were looking at data derived from the Social Networks among Appalachian People (SNAP) study regarding the use of the drug gabapentin in Kentucky.
Gabapentin is an anticonvulsant drug also used to treat certain types of pain. According to the federal government, gabapentin isn’t a controlled substance. But because its potential for misuse has become increasingly evident, some states (Kentucky being the first) have listed gabapentin as a Schedule V drug with the intent of restricting access and curtailing use without a prescription.
But that’s not what happened. After scheduling, Cook noticed that nonmedical gabapentin use was still increasing in the Commonwealth. What had changed, however, was that people were no longer getting gabapentin from their doctors and pharmacies but rather from family, friends and people who sell drugs.
Although the circumstances surrounding Darren’s situation and post-scheduling gabapentin trends in Kentucky are quite different, both point to Cook’s general hypothesis: While tightening access to a drug seems to be an obvious and eminently sensible approach to reducing misuse, scheduling decisions can have unforeseen consequences.
Cook was recently awarded a UK Substance Use Priority Research Area (SUPRA) Graduate Student Grant to explore this hypothesis in her study titled “The Effects of Scheduling on Nonmedical Use of Gabapentin in Kentucky.”
“My study is about gabapentin, but that’s largely because it’s a recently scheduled drug that we can get a lot of data on,” explained Cook. “The study is really about the effect of scheduling.”
Cook’s study has two aims. The first is to determine whether gabapentin-involved overdose deaths changed in Kentucky after it became a Schedule V drug in July 2017. She will accomplish this by comparing data from 12 months before and 12 months after the drug was scheduled.
For the second aim, Cook will collect qualitative data by interviewing 30 Kentucky residents who have taken gabapentin for nonmedical reasons. Using this novel approach of gathering data directly from people who use drugs, she hopes to gauge post-scheduling changes in accessibility, street price, source, frequency and amount of use, and reason for use.
Cook has taught college-level math and statistics for nearly 30 years, and in addition to being a Ph.D. student in the College of Public Health, she is currently a lecturer in the UK College of Arts and Sciences’ Department of Statistics.
With her strong background in statistics, Cook is quite comfortable with the quantitative methods required for her first aim. However, the interview-based approach of the second aim opens new territory for Cook.
“I’ve had to learn a lot about qualitative methods, and it’s exciting to step outside my mathy little world and explore the messy real world. It does seem strange,” Cook joked, “to be doing exactly what I tell my statistics students not to do.”
“But the interviews will be potentially illuminating,” Cook added. “Very few qualitative studies have looked at drug scheduling from the perspective of people who use drugs.”
The findings of her study could — in the best possible way — complicate our picture of the effects of drug scheduling. Ultimately, research such as Cook’s could help reshape scheduling practices to maximize their benefits while minimizing unanticipated and potentially unwelcome consequences.
“You can never tell what the impact of your research will be,” Cook said. “But I’m hoping — my whole family is hoping — that something good will come from this research. Then we all can feel that the tragedy of Darren’s death served as the genesis for positive change.”
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R01DA024598 and R01DA033862. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.




