Open-Drop or Nose Cone Method of Isoflurane Anesthesia in Mice and Rats
PURPOSE: This procedure details how to use an isoflurane/propylene glycol mixture to anesthetize rodents. Isoflurane is an inhalant anesthetic designed for use with a precision vaporizer. This method should be reserved for instances where short duration anesthesia is desired or it is impractical to use a precision vaporizer.
The open-drop method of isoflurane exposure can be used to anesthetize mice and rats for brief non-surgical procedures. To maintain anesthesia for 5-10 minutes duration, a simple nose cone can be constructed from a syringe with the plunger removed.
Note that even when diluted, this method does not allow for more than crude control of the concentration of the anesthetic and therefore the potential for inadvertent overdose with this method is significantly greater than when a precision vaporizer is used. Mice and rats need to be watched very closely and observed during induction to avoid anesthetic overdose and death.
APPLICATION: The open-drop and nose cone method of inducing isoflurane anesthesia is an alternative to the use of a precision vaporizer where short duration anesthesia is desired for minor procedures in rodents. Precision vaporizers provide a much safer and more reliable method of inducing isoflurane anesthesia. The open-drop and nose cone procedures are not acceptable for major surgical procedures or procedures requiring greater than 10 minutes of surgical anesthesia or where the use of a precision vaporizer is required to maintain an adequate and safe level of anesthesia.
Important Safety Consideration: Because of risks to human health, the use of inhalant anesthetic is not recommended on open bench tops. The following procedures should only be performed in a certified ducted hood, biosafety cabinet or portable ductless hood equipped with a charcoal filter. The Department of Environmental Health and Safety should be consulted regarding appropriate scavenging methods to mitigate human exposure to excess anesthetic agent.
PROCEDURES:
Materials:
- Cotton pad (i.e.cotton ball, nestlet or gauze pad)
- Container (with known volume)*
- Wire mesh to fit in bottom of container (using a histology tissue cassette to contain the gauze might be a helpful alternative option to the wire mesh.)
- Mixture of 20%v/v isoflurane** in propylene glycol (for mice)
- Mixture of 30% v/v isoflurane** in propylene glycol (for rats)
- Certified ducted hood, biosafety cabinet or portable ductless hood equipped with a charcoal filter.
Optional Materials:
- To maintain anesthesia for up to 10 minutes duration, a simple nose cone can be constructed from a 3cc syringe (for mice; larger syringe for rats) with the plunger removed or a conical tube (15ml for mice; 50 ml for rats). A pre-cut section of gauze is also required for the nose cone.
Any type of container with a secure lid may be used, provided it is constructed of non-porous material that is sanitizable and allows for constant visualization of the animal. Ensure that the animal does not contact the isoflurane-soaked pad, which can cause skin irritation and potential overdosing since isoflurane is also absorbed through skin. The container should be of sufficient size to comfortably accommodate the animal, but not so large as to require excessive anesthetic.
** Use of isoflurane without propylene glycol dilution is unacceptable, as the vapor pressure of isoflurane will lead to lethal accumulations of anesthetic in the vapor phase.**
Open Drop Procedure (For brief procedures): Mice will remain deeply anesthetized for approximately 30 seconds and rats for one minute. This method can be used for retro-orbital blood sampling, tail biopsies and similar rapid procedures. To maintain longer anesthetic times, see Nose Cone Procedure.
- Don gloves. Open bottle containing appropriate isoflurane/propylene glycol mixture in approved hood. Wet a cotton pad (e.g., nestlet, gauze) with an exact amount of isoflurane mixture. A 1.0 cc volume of appropriate mixture should be used for every 500 cc volume of the anesthesia container.
- Place the cotton pad inside a small container under a wire mesh. The use of the mesh ensures that the animal does not contact the isoflurane-soaked pad, which can cause skin irritation and potential overdosing since isoflurane is also absorbed through skin.
- Place animal in anesthesia jar and close lid tightly. Monitor animal closely. A deep plane of anesthesia is indicated by lack of a righting reflex when the jar is tipped slightly and a respiratory rate that is reduced by ~50% from pre-anesthetic rate (i.e. 80-100 breaths/min). This should take ~1 min for mice and ~2 min for rats. Observe the animal closely so that overdose does not occur.
- Allow animal to remain in deep anesthesia for ~10 sec before proceeding. Remove animal from the anesthesia container and replace lid immediately.
- To ensure an adequate plane of anesthesia, apply a noxious stimulus (i.e. toe pinch) before proceeding with the procedure. If the animal responds to the toe pinch return it to the anesthesia container.
Nose Cone Procedure This method can be used with the Open Drop Procedure above to maintain anesthesia for up to 10 minutes duration and is appropriate for minor surgical procedures, such as subcutaneous tumor implantation. Procedures lasting longer than 10 minutes should be performed using a precision vaporizer system.
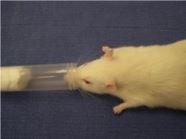
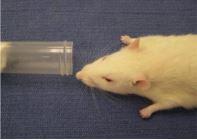
- Slightly moisten the end of a small piece of gauze with the Isoflurane mixture. Insert the gauze into syringe nose cone of appropriate size, with moistened end away from open end of the nose cone. Place prepared nose cone into anesthesia jar, until ready to use.
- Anesthetize the animal as described in steps #1-4 (Open Drop Procedure) above.
- Retrieve the nose cone from the jar, and still working in the hood, place the animal’s muzzle at the edge of the nose cone. Moisten the animal’s eyes with Paralube. Check the depth of anesthesia as described as step #5 above (the depth of anesthesia can be adjusted by moving the animal’s nostrils closer to or further from the end of the cone). Care must be taken not to create a complete seal around the muzzle.
- If appropriate, perform the procedure.
- Allow the animal to recover on a piece of clean paper towel in a bedding-free recovery cage to prevent aspiration injury or death. Monitor the animal closely until it can maintain sternal recumbency, then transfer to the home cage.
- Air dry isoflurane exposed cotton pad(s) and nose cones in the hood for 15 minutes and discard by wrapping in a glove and transferring to a biomedical waste disposal bag or bucket.
Important Safety Consideration: Because of risks to human health, the use of inhalant anesthetics is not recommended on open bench tops. The above procedures should only be performed in a certified ducted hood, biosafety cabinet or portable ductless hood equipped with a charcoal filter. The Department of Environmental Health and Safety should be consulted regarding appropriate scavenging methods to mitigate human exposure to excess anesthetic agent.
**As a reminder DLAR does not have ducted hoods or charcoal filtered hoods in any animal housing room. This method cannot be used in animal housing rooms. Investigators should contact DLAR for locations of appropriately ducted hoods where this can be safely performed in the vivarium procedure rooms.**
For questions or training, please contact any DLAR Veterinarian or the DLAR Training Coordinator.
REFERENCES:
- Itah et al. 2004. A replacement for methoxyflurane (Metofane) in open-circuit anesthesia. Lab.Anim. 38:280-5.
- Nagate, T., T. Chino, et al. (2007). "Diluted isoflurane as a suitable alternative for diethyl ether for rat anaesthesia in regular toxicology studies." Journal of Veterinary Medical Science 69(11): 1137-1143.