UK's Sanders-Brown among 1st locations testing promising new Alzheimer's drug
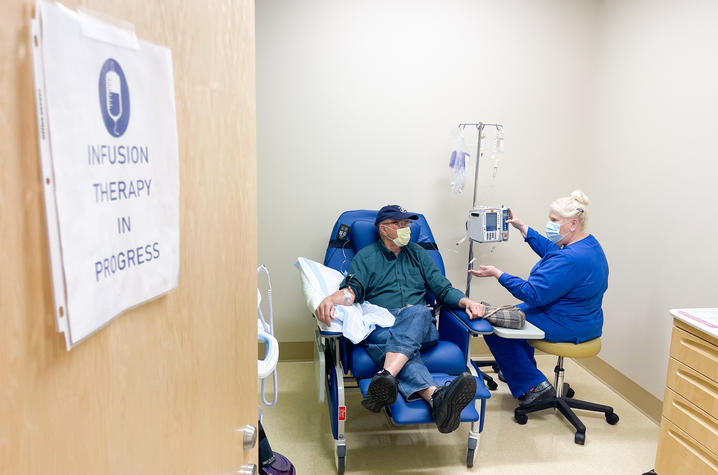
The University of Kentucky is a site for the groundbreaking AHEAD study, the first-ever clinical trial to test the effect of lecanemab (investigational antibody) in people who have no cognitive symptoms of Alzheimer’s disease (AD), but in whom biomarker tests indicate amyloid is present in the brain, known as “preclinical” AD. The AHEAD study is also the first AD trial to recruit people as young as 55 years old who are at risk of developing symptoms of AD as they get older.
In September, the pharmaceutical company Eisai announced positive topline results from Clarity AD, a phase 3 study evaluating the safety and effectiveness of lecanemab, an investigational anti-amyloid beta (Aβ) protofibril antibody for the treatment of mild cognitive impairment (MCI) due to AD and mild AD dementia (collectively known as early AD) with confirmed presence of amyloid pathology in the brain.
Then, just last week, at the International Clinical Trials on Alzheimer’s Disease meeting in San Francisco, Eisai presented the full data from the groundbreaking analysis of the CLARITY study. The results presented demonstrate an almost complete removal of amyloid plaques from the brain of those with early Alzheimer’s that was associated with a 27% slowing of decline. The slowing was seen to start as early as six months and increased over time every three months. Researchers found that treatment showed highly statistically significant changes compared to placebo. Results also show that lecanemab treatment leads to a 31% lower risk of converting to the next stage of disease.
Now, the AHEAD study will test whether the clinical effects reported in the Clarity AD clinically symptomatic population are similar in the AHEAD preclinical AD population.
“When doctors, researchers and scientists from across the globe, including myself, saw the data for the first time, the excitement was contagious. We all realized that for the first time ever in the history of drug development for Alzheimer’s disease, we have broken down the barriers that will one day move us to a cure. Our excitement was propelled by the knowledge that we can succeed eventually in eradicating Alzheimer’s and that even better medicines are on their way”, said Director of Clinical Trials at UK’s Sanders-Brown Center on Aging Greg Jicha, M.D., Ph.D. “Now, moving forward with the AHEAD study, we are testing whether even earlier removal of amyloid plaques can slow or prevent the early appearance of Alzheimer’s disease”
Jicha says at UK they have been working with patients to provide access to lecanemab for over a decade now and have successfully removed the amyloid from the brains of dozens of people.
“We think we are taking this one over the finish line,” said Jicha. “I see hundreds of patients each year that are shocked that they have received the death sentence of Alzheimer’s disease. As of now, there are no cures for this fatal disease that is the sixth-leading cause of death in the U.S. We need to know if we can screen and stop this disease before it destroys the lives of those we love or takes our own lives. The AHEAD study is a path forward."
Looking for a path forward as they age as well as a way to help others are big reasons why many get involved in research at UK.
“I’m retired and just want to help people anyway I can,” said Mike Brown, who participates in the AHEAD study alongside his friend Jim Jackson. The duo travels from Morehead, Kentucky, every two weeks along with their wives for their visit at Sanders-Brown.
“We make a fun little day out of it. We always try to eat somewhere different,” said Sharon Jackson, Jim's wife.
Jim is a retired pediatrician. With his medical background he jumped at the opportunity a few years ago to get involved. “I knew I wanted to be part of it. This is the way science works,” said Jim.
Both Jim and Mike have been in the AHEAD study for several months. That portion, as is the case with most clinical trials, does hold the possibility of being part of the placebo group. They are still blinded for that part of the study so they do not know yet if they had been receiving lecanemab. “You just have to be optimistic and know you are helping others,” said Jim.
However, they did both qualify for an extension of the study, which started just a few months ago. This extension allows them to confidently receive lecanemab for 12 months. Hearing the recent news about the early results for this drug provided an extra dose of enthusiasm for the couples as they continue to make their biweekly drive to Lexington.
“I am just so thankful Jim is part of this because if it prolongs his memory any amount, it is very much worth it,” said Sharon. “Dr. Jicha and the team here at UK are so great and informative. We always have a comfortable experience here. We are so blessed to have a place like this right here in Kentucky. More people should consider getting involved and using the resources offered at Sanders-Brown.”
Jicha says they are proud that UK’s Sanders-Brown Center on Aging is at the forefront of these discoveries.
“You might think such possibilities are limited to the rich and famous; you might think such possibilities are only available in Washington, D.C., New York, Chicago or L.A.," he said. "But you should know that the UK Sanders-Brown Center on Aging is at the forefront of these discoveries, bringing them here to Kentucky long before they may be available to others in the United States.”
The study is focused on making sure the opportunity is available to a wide range of the population. “We especially want to reach out to those that have experienced health care discrimination. If you live in a rural area or are a person of color, you should know that we value you as much as we do everyone else. An effort this important needs to be available to everyone,” said Jicha.
The AHEAD study incorporates innovative features such as screening with plasma biomarkers, novel PET imaging agents, sensitive cognitive outcome scales, dosing tailored to the level of amyloid in the brain, and recruitment approaches to ensure diverse representation. Plasma prescreening and analysis of baseline imaging will be presented at CTAD in November.
Jicha says they truly believe the future of healthy brain aging is within their grasp. “For many, it may be too late for lecanemab to help but we are not giving up on them either. We have over a dozen active experimental medicine trials for anyone at risk, currently experiencing memory loss or even those with more advanced stages of Alzheimer’s disease or other forms of dementia. If you are looking to do more for yourself or a loved one, please reach out to us now.”
The AHEAD study is funded by the National Institutes of Health (NIH) and Eisai Inc., a U.S. subsidiary of Eisai Co. Ltd. (Headquarters: Tokyo), and seeks 1,165 participants from North America. The study has more than 100 study locations worldwide, including North America, Japan, Singapore, Australia and Europe.
To learn more about the AHEAD study, call 1-800-AHEAD-70 or to find a trial site location enrolling near you, visit www.AHEADstudy.org.
Learn more about enrolling in studies at UK’s Sanders-Brown here.
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R01AG054029 and R01AG061848. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The AHEAD study (Clinical Trial number NCT04468659) received funding from NIH and from nongovernmental sources.
This release discusses the investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such an investigational agent will successfully complete clinical development or gain health authority approval.
Credits
Words and Photo: Hillary Smith (Public Relations & Strategic Communication)

