Federal Changes This page will be updated as new information is provided. Researchers, please send federal agency communications you receive or any questions specifically related to awards to ospa@uky.edu and vpr@uky.edu.
Jan. 22: NIH Announces Shift Away from Human Fetal Tissue Research
See below for full announcement.
Jan 13: USDA Research Priorities, Terms and Conditions
See below for full announcement.
Dec. 3: NIH Removes Requirements for Letters of Intent and Unsolicited Applications Requesting $500,000 or More in Direct Costs
See below for full announcement.
Dec. 2: NIH’s Implementation of Common Forms for Due Dates on or after January 25, 2026
See below for full announcement.
Past Announcements
Jan 22: NIH Announces Shift Away from Human Fetal Tissue Research
On Jan. 22, 2026, the National Institutes of Health (NIH) put out notice detailing that NIH will no longer support research using human fetal tissue. See NOT-OD-26-028 on NIH site. The NIH Director also issued a statement: NIH Announces Shift Away from Human Fetal Tissue Research to Accelerate Biomedical Innovation.
Jan 13: USDA Research Priorities, Terms and Conditions
The U.S. Department of Agriculture recently released two programmatic updates on the agency’s research priorities and on the terms and conditions for agency-funded grants.
On December 30, 2025, U.S. Secretary of Agriculture Brooke Rollins signed a Secretary's Memorandum, announcing five new priorities for USDA-funded agricultural research and development aimed at strengthening U.S. agriculture:
- Increasing Profitability of Farmers and Ranchers
- Expanding Markets and Creating New Uses of U.S. Agricultural Products
- Protecting the Integrity of American Agriculture from Invasive Species
- Promoting Soil Health to Regenerate Long-Term Productivity of Land
- Improving Human Health through Precision Nutrition and Food Quality
According to the press release, the memo refocuses research investments on applied outcomes that enhance farmer profitability, productivity, export competitiveness, and lower input costs, while moving away from prior policy approaches viewed as burdensome or misaligned with producer needs.
On December 31, an additional Secretary’s Memorandum was released directing all USDA agencies and staff offices to immediately adopt and implement the first-ever set of USDA General Terms and Conditions for all future awards, effective on all new awards starting December 31, 2025.
Dec. 3: NIH Removes Requirements for Letters of Intent and Unsolicited Applications Requesting $500,000 or More in Direct Costs
Dec. 3, 2025, the NIH issue a notice (NOT-OD-26-019) with two important updates related to application submission policies.
Letters of Intent
Effective immediately NIH will no longer request or accept Letters of Intent (LOIs) as part of the application process. NIH has occasionally requested LOIs within Section IV of the Notice of Funding Opportunity (NOFO) to help Institute, Center and Office (ICO) staff estimate the potential peer review workload and recruit reviewers. An LOI was never required, binding, nor considered during the peer review process. Given NIH’s centralization of peer review processes to improve efficiency and strengthen integrity through the Center for Scientific Review (CSR), the LOI is no longer serving the same purpose to estimate ICO workload. To further increase efficiency and minimize applicant burden, NIH is removing the LOI from the application process. LOIs that have been submitted will not be acknowledged nor reviewed by the respective ICO or CSR. Potential applicants are encouraged to review NOFO instructions carefully as they are subject to change.
Unsolicited Applications Requesting $500,000 or More in Direct Costs
Effective immediately, NIH will no longer require applicants requesting $500,000 or more in direct costs (excluding consortium F&A costs) in any one budget period to contact the funding Institute or Center (IC) before application submission. In line with this change, applicants are no longer required to include a cover letter identifying the Program Official contact which notes that the IC has agreed to accept assignment of the application. All active NOFOs containing this requirement are superseded by this Notice and will be updated going forward. In addition, the NIH Application Guide and Grants Policy Statement will be updated in their next iteration to remove this requirement.
Dec. 2: NIH’s Implementation of Common Forms for Due Dates on or after January 25, 2026
To support strong collaboration between federal research agencies the NIH is adopting the Common Forms for Biographical Sketch and Current and Pending (Other) Support. An NIH notice (NOT-OD-26-018), issued Dec. 2, 2025, provides details for the Common Forms, NIH Biographical Sketch Supplement and instructions required for application due dates and Research Performance Progress Report (RPPR) submissions on or after January 25, 2026. We will share more information on training on these policies and procedures for senior/key personnel as it is available.
Dec. 2: Research Security Training Requirements for NIH
Dec. 2, 2025, NIH put out a notice (NOT-OD-26-017) on NIH implementation of the Research Security Training (RST) requirements outlined in the CHIPS and Science Act of 2022. Each covered individual (defined as senior/key personnel) listed on an NIH grant application must certify that they have completed RST within 12 months of the date of application submission. NIH does not collect Current and Pending (Other) Support at the time of application based on its Just-in-Time policy. NIH will collect the individual certification at the time of the application submission, through the Biographical Sketch in SciENcv.
- Visit the Sponsored Project Services site for more information on SciENcv.
- Visit the SECURE site for instructions on how to complete the Research Security Training (Combined) module through CITI.
At this time, the research security training requirement is optional for NIH. Completion of RST and the individual and institutional certifications will be required for NIH applications submitted for due dates on or after May 25, 2026.
Nov. 24: Updated Guidance on Reopening of NIH Extramural Activities Following the October 1, 2025 - Lapse in Appropriations
Rescheduling October Application Due Dates
All grant applications submitted late for due dates between October 1, 2025, and December 5, 2025, will be accepted through 5:00 PM local time December 8, 2025. There is no additional 2-week late window. This notice applies to all relevant Notices of Funding Opportunity (NOFO), including those that indicate no late applications will be accepted. Institutions need not request advance permission to submit late due to the government shutdown and a cover letter providing a justification is not required.
Post-submission Materials
For applications for January 2026 Council, NIH will accept post-submission materials, as described in NOT-OD-19-083, up to one week before the scheduled peer review meeting.
For all applications for May 2025 Council, NIH will accept a one-page update with preliminary data as post-submission materials for applications submitted under all activity codes, ONLY if the NOFO used for submission allowed preliminary data in the application. One page of preliminary data will be accepted for single component applications or for each component of a multi-component application. The deadline for submitting all post-submission materials, including preliminary data, will be 30 days before the study section meeting. All other materials listed in NOT-OD-19-083 as acceptable post-submission materials will continue to be accepted if submitted 30 days before the study section meeting. Instructions for submission are included in NOT-OD-19-083.
Research Performance Progress Report Submitted to NIH During the Shutdown
Recipients that submitted their Research Performance Progress Reports (RPPR) that were due between October 1 and November 13 should not have to resubmit their reports, unless the institution receives a request from the funding Institute, Center, or Office. If you had submission challenges during the shutdown, contact staff at the appropriate ICO.
Rescheduling Peer Review Meetings
The shutdown required that NIH cancel over 370 peer review meetings, impacting the review of over 24,000 applications. The volume of missed meetings complicates NIH efforts to catch up. In order for these applications to be considered in the January council as originally planned, the following modifications in peer review practices will be made:
- The percent of applications discussed in most meetings will be reduced to 30-35%, instead of the current ~50%.
- Applications voted by the committee to be in the middle third will be designated as “competitive but not discussed” and applications in the lowest third will be designated as “not competitive and not discussed”. Applications in the middle third will be considered for funding, along with the discussed applications.
- Summary statements will be simplified. Narrative paragraphs summarizing committee discussion will not be used. Instead, summaries will have a sentence describing the degree of consensus in the committee vote, plus bullets listing the main score driving points. Summaries for all applications will contain the written critiques from the 3 assigned reviewers, as is currently the case. Summaries for applications that are discussed will have the overall impact score.
The delay in reviewing applications submitted for the January council creates a situation where peer review-related work for the January council will overlap with that of the May 2026 council. For that reason, the modifications in review practices outlined above will be in place through the May 2026 Advisory Council.
Early Stage Investigator Eligibility
NIH will automatically adjust the Early Stage Investigator (ESI) status for applicants whose status has changed during the period of delay caused by the shutdown.
K99/R00 Eligibility
Information updating timelines for the K99/R00 NIH Pathway to Independence Award program will be issued separately.
Loan Repayment Program
The Extramural LRP Application Submission Deadline is now December 4, 2025. Refer to: NOT-OD-26-010 for more details.
Payment Management System
Payments through the Payment Management System (PMS) should be processing as usual. If recipients received a communication during the shutdown that a drawdown request was on hold, please contact PMS directly to determine the status of that pending request.
Financial Operations under a Continuing Resolution
NIH has published a Notice on our financial operations under a continuing resolution within the next few business days. Refer to: NOT-OD-26-011.
See NOT-OD-26-012 on the NIH site.
See the NIH Funding Blog: Emergency Modifications to NIH Peer Review.
Nov. 21: Implementing a Unified NIH Funding Strategy to Guide Consistent and Clearer Award Decisions
There was an important announcement today published on the NIH Extramural News website, titled “Implementing a Unified NIH Funding Strategy to Guide Consistent and Clearer Award Decisions” that announces what factors NIH institutes will consider for funding decisions.
Nov. 18: Reminder of Compliance Requirements for NIH Renegotiated Aims, Objectives, Titles and Abstracts
All changes in scope that were agreed upon by NIH and the Authorized Organizational Representatives (AOR) become new terms and conditions of award, and the recipient must comply with those changes. This includes changes in scope that were renegotiated to align with the agency’s priorities. As such, NIH fully expects recipients to comply with the new terms and conditions, unless otherwise stated in the Notice of Award, or unless NIH has been enjoined by court order from imposing or enforcing such terms while that court order is in effect. If recipients have questions related to the new terms and conditions of the awards, the recipient must seek clarification from the Grants Management Official, prior to drawing down on the funds. Once the funds are drawn down from the HHS payment system, the recipient agrees to the terms and conditions of the award and must fully comply with them. Please be advised that if NIH discovers that recipients have not complied with the new terms and conditions of their award, NIH can immediately take an enforcement action under 2 CFR 200, as appropriate, which includes termination for non-compliance under 2 CFR 200.340(a)(1). For terminations for non-compliance with the revised scope, NIH will provide appeal rights to recipients at the time of the termination and, in accordance with 2 CFR Part 200.340 (c), will report the terminations in SAM.gov, as appropriate. See NOT-OD-26-007 on NIH site for full details.
Nov. 18: NIH Updated Terms and Conditions of Award – Termination and Compliance with Court Orders
Effective October 1, 2025, all new NIH Notices of Award will include the following terms:
- This award is subject to the termination provisions at 2 CFR 200.340. Pursuant to 2 CFR 200.340, by accepting an NIH award, the recipient agrees that continued funding for the award is contingent upon the availability of appropriated funds, recipient satisfactory performance, compliance with the Terms and Conditions of the award, and may also otherwise be terminated, to the extent authorized by law, if the agency determines that the award no longer effectuates the program goals or agency priorities, in line with 2 CFR 200.340(a)(4).
- Any term or condition in this Notice of Award, including those incorporated by reference, that NIH is enjoined by court order from imposing or enforcing, shall not apply or be enforced as to any recipient or subrecipient to which that court order applies and while that court order is in effect.
Additionally, the new Notices of Award will reference 2 CFR 200 instead of 45 CFR Part 75, where applicable. These terms of award will be applied prospectively to new, renewal, supplement, or continuation awards issued on or after October 1, 2025. The updated language will also be incorporated into the FY26 version of the NIH Grants Policy Statement. See NOT-OD-26-009 on NIH site for full details.
Nov. 17: Reopening of NIH Extramural Activities
NIH will be rescheduling all October and November grant application submission deadlines (specific dates to be announced in a future Notice). By delaying due dates that occurred both during the lapse in funding and in the week following, applicants will have access to NIH staff and the help desks as they develop their applications.
Peer review meetings that were scheduled to take place between October 1 and November 14 have been cancelled and will be rescheduled, details to come. Additionally, council meetings that were scheduled to take place between October 1 and November 14 were cancelled and will be rescheduled, details to come.
During the shutdown, the eRA system remained open and available to accept application submissions. As such, eRA is currently reviewing service desk ticket requests from entities and individuals that needed assistance with login and passwords. eRA staff will address the tickets on a first come, first serve basis. Please note that due to the volume the response time may be longer than usual.
The eRA system was also available for recipients to submit Research Performance and Progress Reports, Federal Financial Reports, etc. The email reminders and submission confirmations were held and will be sent out in the coming weeks. If you submitted any reports during this time, no additional action is required unless you are contacted by NIH staff.
For more, see NOT-OD-26-005 on the NIH site.
Nov. 12: Federal Shutdown Ends
After the longest shutdown in history, the House passed a bill Nov. 12 to fund the government through Jan. 30 and President Trump signed it, reopening the federal government, which had been shut since Oct. 1
Sept. 29: CDC Priorities Statement
The U.S. Centers for Disease Control and Prevention (CDC) released a revised priorities statement that includes commitments to:
- gold-standard science and ensuring trust, transparency, and credibility
- global leadership
- ensuring rapid, evidence-based responses to crises
- vaccine safety and efficacy research
- advancing our understanding of the causes of autism spectrum disorder (ASD), neurodevelopmental disorders (NDDs), and chronic disease
- modernizing public health infrastructure and enhancing our approach to health data
Sept. 17: NIH Projects with Foreign Components and NIH Highlighted Topics
NIH has ended the use of foreign subawards to support foreign components. A new application structure for NIH-funded international collaborations was announced on Sept. 12 in NOT-OD-25-155, with a companion forecasted PF5 mechanism NOFO. If you plan to apply for funds to support a foreign collaboration, please review these. Importantly, this new PF5 funding mechanism is not expected to be published until Nov 2025, so you will have to wait until early next year (Jan 25, 2026) to submit one of these new PF5 applications. Between now and then, if you submit a regular investigator-initiated R01 application to support a foreign subaward it will be pulled from the system before being reviewed.
NIH has moved away from Notices of Special Interest (NOSIs) and instead is using a new Highlighted Topics page to indicate areas of interest. This page is anticipated to be updated 3-4 times a year. There are no set aside funds to support projects that touch upon highlighted topics, nor is there a way to indicate your application is being submitted in response to a given highlighted topic. To be determined is whether being responsive to a highlighted topic may help program officers justify select pay nominations at the end of the fiscal year.
August 27: Agency “Gold Standard Science” definitions and implementation plans
August 21: Supreme Court Issues Fractured Ruling on NIH Cuts
In a fractured ruling on August 21, the Supreme Court ruled by a 5-to-4 vote that the Trump administration could for now cancel more than $780 million in grants from the National Institutes of Health that the government said had been intended to explore topics like diversity, equity and inclusion initiatives, gender ideology and vaccine hesitancy. But a different five-justice majority let stand for now a lower court’s ruling that the administration’s underlying policy directing the cuts was probably unlawful and should be put on hold. Read more on that ruling at nytimes.com.
UK community members have free access to digital versions of the New York Times and Wall Street Journal by subscribing through UK Libraries.
August 14: NIH Will Stop Posting Notices of Funding Opportunities in the NIH Guide for Grants and Contracts in FY2026 | NOT-OD-25-143
Since 2005, NIH has posted all grant and cooperative agreement notices of funding opportunities (NOFOs) in both Grants.gov, a federal-wide portal for discretionary funding opportunities, and the NIH Guide for Grants and Contracts (NIH Guide). Both have served as official sources for NIH NOFOs.
This Notice informs the extramural community that, beginning in fiscal year 2026, NIH will no longer post NOFOs in the NIH Guide. Grants.gov will serve as NIH’s single official source for grant and cooperative agreement funding opportunities. The NIH Guide will continue to be used for policy and informational notices.
This effort is part of a wider strategy across NIH to simplify and streamline the application and funding process and to reduce duplication across federal systems.
What to Expect in FY2026
- NIH NOFOs will no longer be accessible from the NIH Guide.
- All NIH NOFOs (expired and active) will remain searchable on Grants.gov using their Classic search or their new Simpler search.
- NOFOs will no longer be included in the weekly NIH Guide Table of Contents subscription emails. You can use Grants.gov subscription services to receive notifications of new NIH funding opportunities.
- NIH will continue to provide tools in the Funding section of NIH Grants & Funding to identify potential funding categories, topics, and opportunities you may be interested in. Any funding opportunity identified through our site will link to Grants.gov for the official opportunity posting.
Resources
August 7: Executive Order Summary: Improving Oversight of Federal Grantmaking
The Executive Order on Improving Oversight of Federal Grantmaking, issued on August 7, 2025, introduces key provisions that will impact how federal research grants are awarded and managed.
Key reforms implemented:
- Enhanced review process: Federal agencies must now designate senior appointees to oversee funding opportunity announcements and awards (grants and contracts). This includes mandatory review by subject-matter experts, interagency coordination to eliminate redundancy, and simplified application language to reduce barriers for smaller organizations.
- New grant criteria: The order establishes specific principles for evaluating grants, including requirements that awards advance presidential priorities and prohibitions on funding activities involving racial preferences, recognition of non-biological sex or support for illegal immigration. It also prioritizes institutions with lower administrative costs and emphasizes measurable outcomes.
- Termination authority: Agencies gain expanded power to terminate grants when they no longer serve agency priorities or national interests. Existing grants must be modified where legally possible to include these termination clauses.
- Administrative changes: The Office of Management and Budget will revise federal grant guidance to streamline applications and limit administrative overhead costs. Agencies must also implement stricter controls on how recipients access and use federal funds.
- Revisions to the Uniform Guidance: The order also calls for Uniform Guidance revisions, which will affect requirements for termination and how administrative costs are covered by federal grants.
Implementation timeline: Agencies have 30 days to report on their current terms and conditions and must hold new funding opportunity announcements until senior appointees and new review processes are in place.
The Office of the Vice President for Research will update the research community as changes are announced by federal agencies in response to this order. See full Executive Order at whitehouse.gov.
July 18: How Does the NIH Initiative to Prioritize Human-Based Research Affect Research Proposing the Use of Laboratory Animals?
In July 2025, NIH announced it will no longer develop new funding opportunities focused exclusively on animal models of human disease. Rather, going forward, new funding opportunities will be designed more broadly with language that also encourages various approaches be considered. This means researchers may choose any model they deem appropriate – including a combination of approaches – to answer a research question when submitting applications seeking NIH support. This strategy is intended to open the possibilities of which types of models can be submitted in response to funding opportunities, not be restrictive or prescriptive. See more detail for this answer at the NIH Q&A.
July 18: Updated Implementation Guidance of NIH Policy on Foreign Subawards for Active Projects
This NIH Notice addresses the implementation of the NIH Policy on Foreign Subawards (See NOT-OD-25-104) for applications submitted before May 1, 2025, and projects active on or before May 1, 2025. NIH recognized the need to identify an alternative approach for removing the foreign subawards from existing grants and cooperative agreements involving human subjects research (e.g., clinical trials and clinical research) at the foreign site. NIH Institutes, Centers, and Offices (ICOs) will have the option to renegotiate the award structure with a recipient such that foreign subawards are financially removed from the primary award and awarded as administrative supplement (i.e., Type 3) awards. Each foreign supplement award will only include funds allocated for a single foreign entity, allowing NIH better ability to track obligations to foreign entities. See full detail in NIH Notice NOT-OD-25-130.
July 18: America First Memorandum for USDA Arrangements and Research Security
A July 8, 2025 Secretarial Memorandum directs the U.S. Department of Agriculture (USDA) to place America First in provisioning all USDA funds, regardless of source and in accordance with any statutory or legal requirements. America First policy avoids the expenditure of American taxpayer funds to help foreign competitors out-produce, out-compete, and out-innovate the United States. For full detail of actions expected from USDA employees and impacts to current and future arrangements, see the memo on the USDA website.
July 17: NIH Guidance on Use of AI and Limit on Applications Per Year
NIH is providing guidance to researchers on the appropriate usage of artificial intelligence (AI) to maintain the fairness and originality of NIH’s research application process. NIH is also instituting a new policy limiting the number of applications that NIH will consider per Principal Investigator per calendar year.
Starting September 25, 2025, NIH will not consider applications that are either substantially developed by AI, or contain sections substantially developed by AI, to be original ideas of applicants. If the detection of AI is identified post award, NIH may refer the matter to the Office of Research Integrity to determine whether there is research misconduct while simultaneously taking enforcement actions including but not limited to disallowing costs, withholding future awards, wholly or in part suspending the grant, and possible termination.
NIH will only accept six new, renewal, resubmission or revision applications from an individual Principal Investigator/Program Director or Multiple Principal Investigator for all council rounds in a calendar year. This policy applies to all activity codes except T activity codes and R13 Conference Grant Applications. Based on recent data, this limit will affect a relatively small number of Principal Investigators while enabling the NIH to maintain consistently high-quality grant application review and appropriately steward taxpayer dollars. See NIH Notice NOT-OD-25-132.
July 10: NIH Funding Announcements to Align with NIH Initiative to Prioritize Human-based Research
On April 29, NIH announced it is prioritizing human-focused research and reducing animal use in research. To further this initiative, all new Notices of Funding Opportunity that relate to animal model systems (NOFOs) must now also support human-focused approaches such as clinical trials, real world data, or new approach methods (NAMs). Examples of NAMs include ex vivo human-based approaches, including perfused human organs and precision-cut tissue slices; in vitro methods, including microphysiological systems and organoids; computational and artificial intelligence-based approaches; and combinations thereof.
Importantly, NIH will no longer issue NOFOs exclusively supporting animal models or limit/specify the types of models that must be used. The intent of this effort is to ensure investigators consider the models most appropriate for understanding human states of health and disease and are not constrained by the NOFO. NIH Institutes, Centers and Program may issue NOFOs that exclude proposals for animal use entirely.
This new emphasis on human-based research will accelerate medical advances, save animals and help NIH achieve its crucial mission of improving human health. Learn more on the NIH site.
July 8: NIH Announces End to Funding for Animal-only Studies
The National Institutes of Health (NIH) announced it will no longer award funding to new grant proposals solely relying on animal testing. The policy was unveiled at the FDA-NIH Workshop on Reducing Animal Testing in comments from Nicole Kleinstreuer, Acting NIH Deputy Director for Program Coordination, Planning and Strategic Initiatives. New grant proposals will require applicants to utilize computational modeling, artificial intelligence (AI) or “organs-on-a-chip” technologies to determine human health impact. More information will be shared as it becomes available and the NIH issues notices. Read more in Drug Discovery & Development.
July 3: Update on Federal Judge’s June Ruling on NIH Grant Cuts
On June 16, a federal district judge in Massachusetts ordered restoration of hundreds of National Institutes of Health (NIH) grants that focused on the health of Black communities, women and L.G.B.T.Q. people. (Read more on that ruling at nytimes.com.) The fate of these NIH grants is still in litigation. There is no information at this time on how grants to UK will be affected. Please be patient as this issue moves through the legal system.
UK community members have free access to digital versions of the New York Times and Wall Street Journal by subscribing through UK Libraries.
June 23: Federal Judge’s Ruling to Reinstate NIH Grants
A federal judge has ordered the National Institutes of Health to reinstate hundreds of research grants that were canceled under the Trump administration. The judge ruled the terminations — many affecting studies on race, gender identity, LGBTQ health and related topics — were discriminatory and lacked legal justification. The order is preliminary and may be appealed. There are no direct immediate impacts.
June 12: NIH Notice of Rescission of Civil Rights Term and Condition of Award
Effectively immediately, NIH rescinds Guide Notice “Notice of Civil Rights Term and Condition of Award” (NOT-OD-25-090). NIH is awaiting further federal-wide guidance and will provide a future update to the extramural community. The previous guidance is no longer in effect. This Notice only rescinds the language in NOT-OD-25-090 and does not rescind current policies related to existing laws, regulations or other HHS or NIH policies that are outlined in the NIH Grants Policy Statement. See NIH NOT-OD-25-124
May 27: DoD 15% Cap Implementation Communications
We are aware that some researchers received messages last week from Department of Defense (DoD) program managers indicating immediate implementation of a new DOD F&A 15% rate. According to Department of Justice (DOJ), the rate is not currently in effect, and DoD has “sent out a message instructing its grants community that the 15% rate is not effective immediately, and that DoD components should ensure that premature implementation of Secretary Hegseth’s memo does not occur.” If a program manager or other agency officer reaches out to you, please forward that communication to ospa@uky.edu and vpr@uky.edu to officially respond.
May 22: Why Did NIH Expire Some Funding Opportunities Because of the Simplified Review Framework, and What Does It Mean for My Application?
The simplified review framework went into effect earlier in 2025, and NIH has been busy preparing staff, peer reviewers and study section chairs for its arrival at summer 2025 review meetings. NIH has since identified some Notices of Funding Opportunity (NOFOs) that were not expired or reissued as indicated in the implementation plan shared in early 2024. To avoid confusion and reduce reviewer burden at review meetings, NIH has expired those NOFOs (see NOT-OD-25-113). Applications already submitted under the expired NOFOs will be reviewed in summer 2025 review meetings under the simplified review framework (see NOT-OD-25-116). Applicants who were planning to apply to the expired NOFOs for future review cycles should instead consider applying through a suitable parent announcement. View more Q&A on the NIH site.
May 20: NSF Deferred Implementation of Indirect Cost Policy for Institutes of Higher Education (IHEs)
The National Science Foundation (NSF) is temporarily pausing implementation of NSF Policy Notice: Implementation of Standard 15% Indirect Cost Rate (NSF 25-034) through June 13, when a hearing is scheduled to occur in the United States District Court for the District of Massachusetts in Association of American Universities, et al. v. National Science Foundation, et al., No. 25-cv-11231. This policy applies to all new awards and associated subawards to Institutions of Higher Education (IHEs). New NSF awards and associated subawards issued during this pause will not implement NSF 25-034 but will include a term applying NSF 25-034 for the entirety of the award if there is a court decision permitting application of the policy. NSF reserves the right to modify, rescind, or fully implement NSF 25-034 to the entirety of awards and subawards issued to IHEs on or after May 5, 2025 in accordance with applicable legal rulings and administrative procedures.
May 15: Federal Judge Blocks DOE 15% Cap
A federal judge in Massachusetts granted an injunction to block the Department of Energy (DOE) from drastically reducing funding that helps universities conduct advanced research, including on quantum computing and renewable energy. The decision involved a proposed cap of 15 percent on indirect costs. Read more on nytimes.com. UK community members have free access to digital versions of the New York Times and Wall Street Journal by subscribing through UK Libraries.
May 15: Notice of Early Expiration of Notices of Funding Opportunities
On October 19, 2023, NIH announced a new framework for the peer review of most research project grant (RPG) applications. NIH identified a number of Notices of Funding Opportunities (NOFOs) that were not expired or reissued as indicated in the implementation plan outlined in NOT-OD-24-085. Consequently, NIH will expire the listed NOFOs at the end of the current application cycle (May 24, 2025). NIH will accept R01, R21 and R34 continuous submission applications intended for the standard May 7, 2025 AIDS due date through June 1, 2025 (end of the standard continuous submission receipt period). No applications for due dates on or after May 25, 2025 will be accepted. See NIH notice NOT-OD-25-113 for full list of expired NOFOs.
May 9: DoD 2025 Decision Matrix Update
The Department of Defense (DoD) has published the 2025 Decision Matrix to Inform Fundamental Research Risk Decisions. These updates will be effective for all proposals submitted on or after May 9, 2025.
May 8: Grant Re-budgeting Requests
If you are approached by a program officer to re-budget or change grant agreement terms, please do not engage with the agency. Immediately alert Collaborative Grants Services (CGS) and Office of Sponsored Projects Administration (OSPA) so that we can develop an institutional response. Only OSPA and the Institutional Official have the authority to negotiate with sponsors.
May 7: Updated NIH Processes for No-Cost Extensions
NIH has temporarily disabled the No-Cost Extension (NCE) functionality in eRA Commons. The Director of NIH has directed NIH staff to review all existing grants and cooperative agreements to ensure that NIH awards do not fund off-mission activities or projects. Therefore, temporarily disabling the NCE functionality in eRA Commons will allow NIH staff to review and assess all NCE requests to confirm that the activities proposed during the extension align with the NIH mission and agency priorities. At this time, all requests for NCEs must be submitted as a prior approval request in eRA Commons, for NIH review and approval. Requests for activities that do not align with the NIH mission and agency priorities will not be approved. View NOT-OD-25-110 on NIH site.
May 2: Indirect Rates for NSF Grants
On Friday, May 2, the Trump administration instituted a 15% cap on indirect rates for National Science Foundation (NSF) grants, effective on all new awards beginning May 5. You can read more about that order on the NSF site here. The University of Kentucky will submit new NSF proposals at the currently negotiated rate of 54% but will also note that the University will accept the 15% if it is ultimately implemented. On May 3, the Association of Public and Land Grant Universities – of which the University of Kentucky is a member – filed a lawsuit seeking to block this order. As that litigation progresses, we will update this information.
May 1: Certificates of Confidentiality for Research Not Funded by NIH
The NIH Certificates of Confidentiality (CoC) system is temporarily unavailable while undergoing renewal of its Paperwork Reduction Act (PRA) approval. Currently, NIH cannot accept submissions to the CoC system or Institutional Official verifications. An update will be provided by July 2025. Note: CoC for NIH-funded studies (i.e., deemed issued CoC) are not affected. View CoCs NIH site.
May 1: Updated NIH Policy on Foreign Subawards
Effective May 1 until the details of the new foreign collaboration award structure are released, the National Institutes of Health (NIH) will not issue awards to domestic or foreign entities (new, renewal or non-competing continuation), that include a subaward to a foreign entity. Additionally, NIH will no longer accept prior approval requests to add a new foreign component or subaward to an ongoing project. In all cases, NIH will allow Institutes, Centers and Offices (ICOs) to renegotiate awards, whether new, renewal or non-competing, to remove subawards to foreign entities and, where the work can be performed domestically, allow the funds to be re-budgeted for use by the prime recipient (domestic or foreign) or a domestic subrecipient. If a project is no longer viable without the foreign subaward, NIH will work with the recipient to negotiate a bilateral termination of the project, taking into consideration any need to support patient safety and/or animal welfare. View full notice: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-25-104.html.
April 21: NIH Issues New Civil Rights Term and Condition of Award
This Notice was cancelled as of 6.12.25
The National Institutes of Health (NIH) issued a Notice, effective immediately, with a new Civil Rights term and condition that modifies the current terms and conditions for all grants, cooperative agreements and other transaction (OT) awards. View the Full notice on the NIH site.
April 18: NSF Updated Priorities Related to Broader Impacts
The National Science Foundation (NSF) continues to review all projects using Intellectual Merit and Broader Impacts criteria. NSF’s broadening participation activities, including activities undertaken in fulfillment of the Broader Impacts criterion, and research on broadening participation, should not preference some groups at the expense of others or directly/indirectly exclude individuals or groups. Research projects with more narrow impact limited to subgroups of people based on protected class or characteristics do not effectuate NSF priorities. Learn more at NSF.gov.
April 16: Federal Judge Blocks Cuts to DOE Indirect Costs
A federal judge has issued a temporary restraining order, temporarily blocking the Department of Energy (DOE) from cutting its support of indirect costs to 15%.
April 11: DOE to Reduce Indirect Costs to 15%
In a new policy memorandum shared with grant recipients at colleges and universities, the Department of Energy (DOE) announced that it will limit financial support of “indirect costs” of DOE research funding to 15%. Learn more at energy.gov
- The Association of Public and Land-Grant Universities (APLU) – of which UK is a member – along with the Association of American Universities (AAU) and American Council on Education (ACE) filed litigation on April 14 challenging this action. Read more about the litigation here.
April 1: Major Layoffs at Health Agencies
Health Secretary Robert F. Kennedy Jr. announced last week that he was shrinking his department by 10,000 employees. Some senior leaders based in the Washington, D.C., area, including leaders at the FDA, CDC and NIH, received notices that they were losing their jobs. Read more on nytimes.com. UK community members have free access to digital versions of the New York Times and Wall Street Journal by subscribing through UK Libraries.
March 25: NIH Common Forms Implementation for Biosketch and Current and Pending (Other) Support
To further support a successful transition to the Common Forms, NIH is postponing the May 25, 2025 implementation for all applications and Research Performance Progress Reports (RPPRs). NIH will issue future Guide Notices outlining the new effective date and details as they are finalized. NIH applicants and recipients must continue to use the current NIH Biosketch and Other Support format pages for applications, Just-in-Time (JIT) and RPPRs. Read more from NIH.
March 11: Consolidation of study sections under Center for Scientific Review
Announced late last week, NIH is planning to move all of its study sections under the oversight of the agency’s Center for Scientific Review (CSR). Under the new plan, CSR would conduct all first-level reviews, eliminating institute-based study sections. NIH said this cost-cutting effort was planned before the change in administration occurred. CSR has begun posting study section meeting notices to the Federal Register. Read more from NIH.
March 7: Foreign Assistance Review emails
We are aware that some UK investigators have received emails requesting they undertake a “Foreign Assistance Review” from federal agencies. If you receive a such an email, please do not respond but instead send that federal agency communication and any other questions specifically related to research awards to ospa@uky.edu and vpr@uky.edu and those offices will communicate with you to coordinate any required response.
Feb. 26: NIH resumes Federal Register notices
The NIH released a statement saying the agency could now "begin sending notices incrementally to the Office of the Federal Register to advertise meetings of scientific review groups/study sections and begin their resumption." The agency planned to submit Federal Register Notices for the next 50 meetings, according to the statement. That will allow for the first phase of grant application reviews to start to resume. But Federal Register notices for other types of meetings remain "on hold," which means the later stages of grant review remain frozen.
Feb. 21: Federal Judge Extends Pause on Cuts to NIH Indirect Costs
A federal judge has kept in place an order blocking the administration from implementing a cap on how much indirect costs the National Institutes of Health pays grant recipients. The temporary restraining order was set to expire on Monday, Feb. 24, and has been extended until U.S. District Judge Angel Kelley can make a final decision on whether to issue an injunction on the proposed change. Read more on nytimes.com. UK community members have free access to digital versions of the New York Times and Wall Street Journal by subscribing through UK Libraries.
Feb. 19: Federal Register hold on submissions
We have been made aware that there is a hold on submissions to the Federal Register, where public notices of upcoming meetings — including NIH study sections — are required by law to be posted. We are also aware that some study sections have been cancelled. We are monitoring the situation and will provide more detail when available. Read more on thetransmitter.org.
Feb. 15: Update from NIH to study section reviewers
To align with new administration guidance:
- Do not evaluate, score or factor into final scoring the Diversity Plan section.
- Reviewers must evaluate as usual the scientific justification for Inclusion of Women, Minorities and Individuals Across the Lifespan in applications with human subjects (excluding research that qualifies for Exemption 4).
- Reviewers must evaluate as usual SABV (sex as a biological variable) in vertebrate animal and human studies.
Feb. 12: National Institute of Justice (NIJ) guidance on projects that ask about “gender”
NOTE: This guidance currently only applies to National Institute for Justice (NIJ) grants. However, other federal grantmaking entities may issue similar directives in the future.
Consistent with the Executive Order (EO) entitled Defending Women from Gender Ideology Extremism and Restoring Biological Truth to the Federal Government (Defending Women), the U.S. Department of Justice’s National Institute of Justice (NIJ) is providing the following instruction and procedures and is seeking award information on or related to surveys, forms, or other data collection tools that ask about “gender” or “gender identity.”
Instruction. If an award-funded project includes a survey, form, or data collection tool that asks about “gender” or “gender identity,” it must instead ask about “sex,” with only two available responses: “male” and “female.” A “choose not to disclose” response is not permitted. All questions about “gender identity” must be removed. Therefore:
- If the project’s survey/form/data collection tool is under development or will be under development later in the project timeline:
- Ensure that the question complies with the above Instruction.
- If the project’s survey/form/data collection tool has been developed, but has not yet been administered:
- If it has been reviewed and approved by the Institutional Review Board (IRB) but not by NIJ’s Human Subjects Privacy (HSP) team, change the question(s) to be in compliance with the above instruction before submitting the package to NIJ for HSP review.
- This may require resubmission for review and modification approval by the IRB.
- If it has not yet been approved by the IRB, change the question(s) to comply with the above Instruction before submitting the package for IRB and NIJ HSP review.
- If it has been reviewed and approved by the Institutional Review Board (IRB) but not by NIJ’s Human Subjects Privacy (HSP) team, change the question(s) to be in compliance with the above instruction before submitting the package to NIJ for HSP review.
- If the project’s survey/form/data collection tool has been developed, approved, and administered:
- If administration is ongoing, pause administration and change the question(s) to comply with the above Instruction.
- This may require resubmission for review and modification approval by the IRB.
- Submit the updated package to NIJ for HSP review and approval. Administration of the survey/form/data collection may resume only upon the award recipient’s receipt of notice of NIJ approval.
- If administration is ongoing, pause administration and change the question(s) to comply with the above Instruction.
Your timely compliance is appreciated. Failure to comply with this directive may affect the award.
Feb. 11: Judge Halts Cuts to NIH Indirect Costs
A federal judge in Boston ordered a nationwide temporary pause on plans by the National Institutes of Health to substantially slash indirect cost reimbursements for research to universities, medical centers, and other grant recipients. Read more on statnews.com.
Feb. 10: NIH Notice on Indirect Cost Rates
The National Institutes of Health (NIH) issued guidance on Feb. 7 regarding cuts to federal research grants. Specifically, the NIH announced it would cap the rate for what are known as “indirect costs” to 15% for all existing and new NIH contract awards. On Feb. 10, litigation was filed by multiple states challenging this decision.
If this policy change is enacted, it will impact the way we do research at the University of Kentucky. It will cost UK tens of millions of dollars annually and will hit our local and state economies. More important than any numbers, though, it will impact the work we do to advance the health of Kentucky in those areas most critical to our future — including cancer, heart disease, Alzheimer’s and substance use disorder.
How the university is responding:
- Our government relations team is meeting with our congressional delegation and others to communicate how fundamentally important and serious this issue is to our community and all those we serve through discovery and healing.
- Our cabinet, deans and other leaders are meeting regularly to confer on this and other issues to ensure we remain careful and prudent stewards of our resources — that we are moving thoughtfully to protect what we do and the future of our research enterprise.
- We will comply with all federal and state laws and policies. That is our responsibility. At the same time, we will forcefully advocate for what we do and our vital mission to advance this state. This includes advocating for the restoration of these critical research dollars.
- We will continue to keep you informed of important updates.
- See President's message: Potential Impact of Federal Research Cuts
Feb. 5: eRA Commons Extension Unavailable
We typically extend NIH awards using the "Extension" option in eRA Commons. The extension option is currently not available. We checked several awards that should be eligible for an extension and the extension option wasn’t available. eRA Commons confirmed that further guidance is pending. In the meantime, we need to submit No-Cost Extension (NCE) requests directly to the NIH Grants Management Specialist (GMS). Please work with CGS and OSPA post-award staff to submit NCE requests via this new mechanism.
Feb. 4: NIH Study Sections Resume
The National Institutes of Health (NIH) resumed some study sections to review grant applications. An NIH internal notice announced that as of Monday, Feb. 3 advisory councils are now permitted to hold closed sessions to discuss and approve funding for research projects, but not open sessions involving public presentations or discussions. See STAT article.
General Award Guidance
Unless you have received a notice to suspend activities for a specific award from a sponsor, you can continue your research activities.
Indirect Costs FAQ
How do indirect costs impact UK?
The NIH is the largest funder of health research in America. In the past five years the University of Kentucky — as a major health research and clinical institution — received an annual average of $159 million in NIH awards (grants and contracts).
Those awards fund basic science research into the diseases and illnesses that most impact Kentucky: cancer, diabetes, heart disease, children’s health, aging-related illness, opioid use disorder and many others.
Awards have two major components. There are direct costs — dollars directly associated with the scientific research in question.
And there are indirect costs — what are often called Facilities and Administration or F&A. Those are dollars associated with the award to pay for items that make that research possible. That could be the construction and outfitting of a lab, research equipment, utilities such as ventilation, heat and lighting, associated technology and graduate students who work in the lab setting.
That indirect cost or rate is negotiated for a period of time between an institution — like UK — and the NIH. Those rates range from 20% of the cost of a grant to 54%, depending upon the research being conducted and the terms of the award.
For example, a $1 million grant with a 37% indirect rate would mean that $1 million directly funds research; $370,000 ultimately goes to the university to allocate to pay those support costs, like facilities, equipment, technology and personnel. The structure for how those dollars can be used is very prescribed.
Cutting the rate to 15% — what the NIH has described as a benchmark for what private foundations that award grants provide — would cut tens of millions of dollars in essential support services to scientists and clinicians who are asking the most important questions about the biggest health challenges Kentucky faces. This one change, if enacted for the next 12 months, would represent a cut of at least $40 million to the University and its critical research efforts on behalf of the health of our state.
Too, the comparison between a private foundation providing a grant around research in education policy, for example, simply does not involve the same cost or cost structure as a basic science grant that could include building and lab space and all the supports that go along with that infrastructure. The complicated discovery and research our investigators perform cost more than the research often funded by private foundations.
Where can I find more resources about F&A?
The Council on Government Relations (COGR) has many resources on its website.
Notices from Agencies
-
"Gold Standard Science" Definitions and Implementation Plans (8.26.25)
-
Updated Implementation Guidance of NIH Policy on Foreign Subawards for Active Projects (7.18.25)
- America First Memorandum for USDA Arrangements and Research Security (6.18.25)
- Supporting Fairness and Originality in NIH Research Applications (6.17.25)
-
NIH Notice of Rescission of Civil Rights Term and Condition of Award (6.12.25)
-
RESCINDED: NIH Notice of Civil Rights Term and Condition of Award (4.12.25)
-
Common Forms for Biographical Sketch and Current and Pending (Other) Support (3.26.25)
-
Supplemental Guidance to the 2024 NIH Grants Policy Statement: Indirect Cost Rates: NOT-OD-25-068 (2.7.25)
-
Office of Management and Budget (OMB) has rescinded M-25-13 (1.29.25)
-
Message from the National Science Foundation (NSF) (1.28.25)
-
Q&A from the Office of Management and Budget (OMB), on temporary pause (1.28.25)
-
RESCINDED: Memorandum from the Office of Management and Budget, Temporary Pause of Agency Grant, Loan, and Other Financial Assistance Programs (1.27.25)
-
Pause on public communications from Department of Health & Human Services (DHHS) (1.21.25)
Messages from the President
-
July Update on Federal Actions (7.7.25)
-
May Update on Federal Actions (5.23.25)
-
Our Principles and Priorities as Kentucky’s University (4.29.25)
-
Update on Federal and State Issues (3.19.25)
-
Update on Federal Policy Changes 2 (1.29.25)
-
Update on Federal Policy Changes (1.28.25)
-
Monitoring Federal Policy Changes (1.24.25)
Messages from the Vice President for Research
-
Resilience in Research (2.27.25)
Additional Resources & Contacts
- University of Kentucky Monitoring Federal Changes 2025
- Council on Government Relations (COGR) 2025 Administration Transition Information & Resources (cogr.edu)
- Association of Public & Land-Grant Universities (APLU) (aplu.org)
Contacts
- Researchers, please send federal agency communications you receive or any questions specifically related to awards to ospa@uky.edu and vpr@uky.edu.
- Employees, please communicate with your supervisors, directors, deans and unit administrators, who should elevate questions and concerns to the appropriate university leaders.
- Leaders and unit supervisors who have questions of law, specifically, should go to the General Counsel.
- Students, please reach out to the Office for Student Success.
- General questions can go to govquestions@uky.edu.
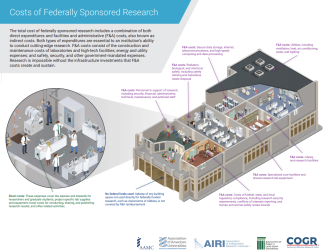
Costs of Federally Sponsored Research
Click below to download a PDF from COGR providing a comprehensive look at the costs of federally sponsored research.