UK researchers find Alzheimer’s-like brain changes in long COVID patients
New research from the University of Kentucky’s Sanders-Brown Center on Aging shows compelling evidence that the cognitive impairments observed in long COVID patients share striking similarities with those seen in Alzheimer’s disease and related dementias.
The study, published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, highlights a potential commonality in brain disorders across these conditions that could pave the way for new avenues in research and treatment.
The study was a global effort, funded by a multitude of grants from the U.S. National Institutes of Health, the Alzheimer’s Association and international organizations. The project also brought together experts from various fields of neuroscience.
Researchers at the UK College of Medicine led the study, including Yang Jiang, Ph.D., professor in the Department of Behavioral Science; Chris Norris, Ph.D., professor in the Department of Pharmacology and Nutritional Sciences; and Bob Sompol, Ph.D., assistant professor in the Department of Pharmacology and Nutritional Sciences. Their work focuses on electrophysiology, neuroinflammation, astrocytes and synaptic functions.
“This project benefited greatly from interdisciplinary collaboration,” Jiang said. “We had input from experts, associated with the Alzheimer’s Association International Society to Advance Alzheimer's Research and Treatment (ISTAART), across six countries, including the U.S., Turkey, Ireland, Italy, Argentina and Chile.”
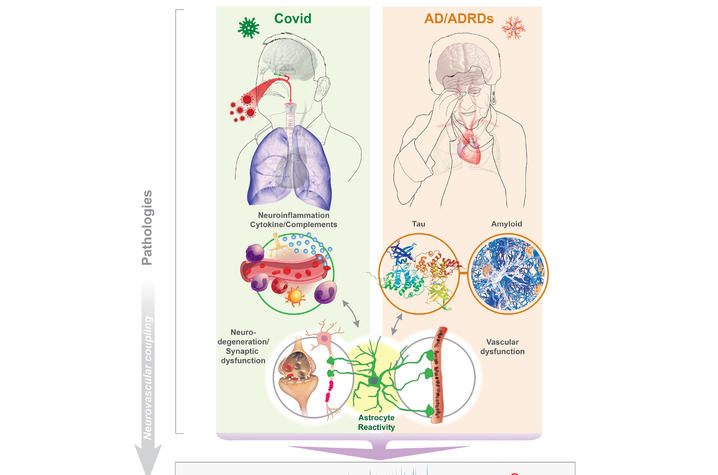
Jiang and the collaborative team focused their work on understanding the “brain fog” that many COVID-19 survivors experience, even months after recovering from the virus. This fog includes memory problems, confusion and difficulty concentrating. According to Jiang, “the slowing and abnormality of intrinsic brain activity in COVID-19 patients resemble those seen in Alzheimer’s and related dementias.”
This research sheds light on the connection between the two conditions, suggesting that they may share underlying biological mechanisms. Both long COVID and Alzheimer’s disease involve neuroinflammation, the activation of brain support cells known as astrocytes and abnormal brain activity. These factors can lead to significant cognitive impairments, making it difficult for patients to think clearly or remember information.
The idea that COVID-19 could lead to Alzheimer’s-like brain changes is a significant development.
“People don’t usually connect COVID-19 with Alzheimer’s disease,” Jiang said, “but our review of emerging evidence suggests otherwise.”
The publication in Alzheimer’s & Dementia reveals that the cognitive issues caused by COVID-19 reflect similar underlying brain changes as those in dementia.
The study’s insights emphasize the importance of regular brain function check-ups for these populations, particularly through the use of affordable and accessible tools like electroencephalography (EEG).
The study not only highlights the shared traits between long COVID and Alzheimer’s, but also points to the importance of further research.
“The new insight opens avenues for future research and clinical practice, particularly in studying brain oscillations related to neural biomarkers of mild cognitive impairment in people with long COVID,” said Jiang.
One of the key findings is the role of astrocytes — support cells in the brain that have not been as thoroughly studied as neurons. The research suggests that damage or activation of these cells by COVID-19 can cause synaptic dysfunctions, leading to the abnormal brain activity observed in both conditions. This discovery is significant because it may help explain why EEG patterns in COVID-19 patients resemble those seen in the early stages of neurodegenerative diseases like Alzheimer’s.
Researchers believe this work could have a direct impact on patient care. They are advocating for routine EEG exams to detect early brain changes in both COVID-19 survivors and those at risk for cognitive decline.
“EEG patterns in COVID-19 patients resemble those seen in early neurodegenerative diseases,” said Norris.
“These similarities may be due to shared issues such as brain inflammation, astrocyte activity, low oxygen levels and blood vessel damage,” said Sompol.
By detecting these changes early, health care providers could potentially identify at-risk individuals sooner and implement interventions to prevent or slow the progression of cognitive decline.
As research continues, the team is particularly interested in how EEG monitoring can predict long-term outcomes in COVID-19 patients and assess the effectiveness of treatments aimed at preventing cognitive decline.
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers P30AG072946, P01AG078116 and R56AG060608. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
More from this series Research Priorities - Neuroscience
Credits
Words: Hillary Smith (Public Relations and Strategic Communications)
Research illustration by Tom Dolan.


